The FDA’s Sentinel System Monitors Use, Safety, and Effectiveness of Medical Products
By Aimee Lee Russell, Associate, Programs
On Wednesday, Aug. 21, Danijela Stojanovic, PharmD, PhD, an epidemiologist at the Center for Drug Evaluation and Research with the Food and Drug Administration (FDA) and Mr. Stephen Mikita, JD, Patient Representative and Lead, Sentinel Engagement Partners Workgroup, participated in a discussion of the “Sentinel System – FDA’s Tools for Assessing Medical Product Safety and Gathering Real-World Evidence.”
Dr. Stojanovic described how the Sentinel System was created in response to a U.S. Congressional mandate to monitor the use, safety, and effectiveness of medical products. In 2016, Sentinel launched the Sentinel Distributed Database (SDD), a resource of real-world evidence to support regulatory actions to protect public health. The data have helped inform health care provider decision-making with patients. Patients can engage with the SDD by serving on clinical and scientific advisory boards, attending Sentinel’s public workshops and meetings, and visiting Sentinel’s website for news and updates.
Mr. Mikita shared how the Sentinel Engagement Partners Workgroup was established to share the work the Sentinel Initiative does with important stakeholders. The Sentinel Engagement Partners Workgroup developed narratives customized for target constituencies such as the general public, patient advocacy groups, and providers. For example, the Workgroup changed the Sentinel story infographic describing what the Sentinel System does to make it more accessible to patients.
Before
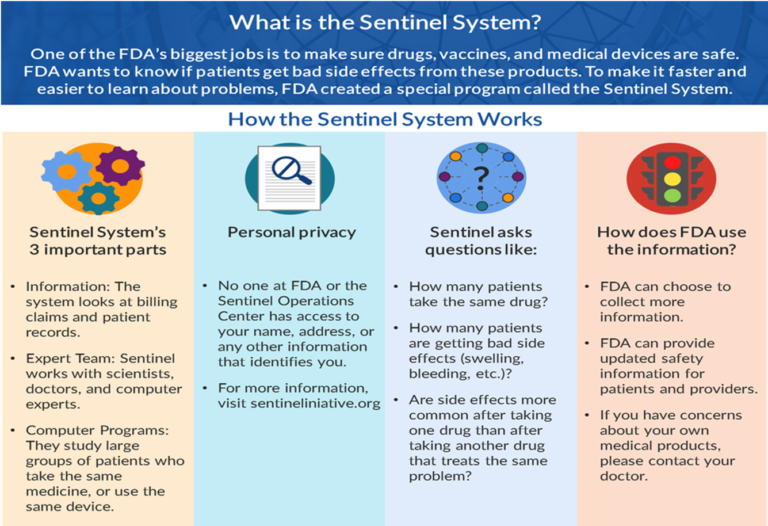
After

The Sentinel Engagement Partners Workgroup also created a social media toolkit, including the updated infographic, a Patient Advocacy Group Narrative, and FAQs for Patient Advocacy Groups.
Please listen to Dr. Danijela Stojanovic’s and Mr. Stephen Mikita’s discussion here for more information about the Sentinel System and the Sentinel Engagement Partners Workgroup.
